Now, we will learn how to write configurations of neutral atoms.
1. Figure out how may electrons there are. (look at atomic number) Start at 1s and keep adding until there are none left.
2. When drawing arrows to show electron spins, always draw the upwards first, then downwards. You can have more upwards than downwards. (if this is confusing, read on and we will explain later)
For example, let's do Na.
Na has the 11th atomic number, and in a neutral atom, it has 11 electrons.
We always start with 1s. If we count to 11, we realize that there will be 2 in 1s, 2 in 2s, 6 in 2p, 1 in 3s, because 2+2+6+1 = 11.
If we want to visualize it:
1s 2s 2p 3s
↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑
Each arrow represents one electron, and counting the arrows, there are 11.
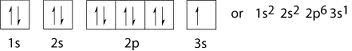
Notice that the electron in 3s is not paired because there are not enough arrows.
In written form, we would write the configuration as
1s2 2s2 2p6 3s1
The superscript just tells us how many electrons are in each subshell. Notice that the first 3 subshells are full, and the last one (3s) just has 1 electron. If the element was Magnesium, then it would be full, since magnesium has one more electron than sodium.
Now, let's write some configurations for ions. This is pretty similar to above, but it just involves one extra step. For example, if we were given Cl-
The - just means that we need to gain an extra electron to make it full. First, just do the electronic configuration normally, as if the - charge did not exist. (in other words, do it for 17 electrons)
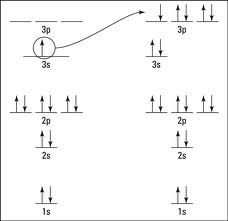
We would get 1s2 2s2 2p6 3s2 3p5. In arrows, it would be:
1s 2s 2p 3s 3p
↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓
The 3p is not full, as there is one more spot available. Now, we look back at the charge. - means to add an electron, so we would add one to the last unfilled subshell. (in red)
For a + charged ion, we would still write it out as if it were neutral, but then removing electrons from the outermost.
Now what if we were asked to do the configuration of an element with an extremely high atomic number? It would take forever! There's a shortcut called Core Notation.
The set of electrons can be divided into two subsets: core and outer. The core of the atom is the set of electrons with the configuration of the nearest noble gas with an atomic number lower than the element in question.
The outer is the electrons outside the core. The core electrons normally take part in chemical reactions.

Step 1: locate the noble gas above the element. If we had Nickel, we would not use Krypton, even though it is closer. We would use Argon instead.
Step 2: Square bracket the noble gas configuration.
Step 3: Start from the noble gas and add electrons until you reach the element.
For example, Chlorine.
The noble gas above chlorine is Neon, which has 10 electrons.
In written form, the configuration for chlorine would be:
1s2 2s2 2p6 3s2 3p5. Now, we can replace the first 10 electrons with Ne. This means that 1s2 2s2 2p6 will be replaced.
Core Notation for Chlorine: [Ne] 3s2 3p5
That seems so much simpler and cleaner than the entire written configuration, right?
However, you don't want to write it out fully, and then replace it. Instead, realize that Ne goes up to 2p6, and just write [Ne] first, then add the remaining electrons.
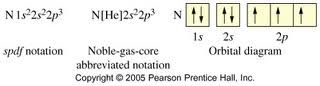
There are two exceptions for electronic configurations.
In copper, you would expect 1s2 2s2 2p6 3s2 3p6 4s2 3d9 or [Ar] 4s2 3d9. However, it is actually 1s2 2s2 2p6 3s2 3p6 4s1 3d10 or [Ar] 4s1 3d10. This is because copper likes to gain stability will a full d-subshell.
In chromium, we expect [Ar] 4s2 3d4, but instead, we actually get [Ar] 4s1 3d5, because chromium likes to gain stability with a half-full d-subshell.
Here's a worksheet:
http://www.chemteam.info/Electrons/WS-Configs&light.pdf
http://www.everettcc.edu/uploadedFiles/Student_Resources_and_Services/TRIO/Electron_configurations_wksht.pdf
And a video!
It is really a helpful blog to find some different source to add my knowledge. I came into aware of new professional blog and I am impressed with suggestions of author.FORTIAP 28C
ReplyDelete