What we mean by open shell is that the shell contains less than the maximum number of electrons.
In contrast, a closed shell would be completely full.
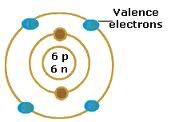
The valence electrons are all the electrons in the atom except in core and filled d/f subshells. In other words, the valence electrons are the s, p types of the outer set, and ONLY unfilled d and f subshells.
For example, if we were given the configuration of Calcium as:
1s2 2s2 2p6 3s2 3p6 4s2
To see the valence electrons easier, put that configuration in Core Notation:
[Ar] 4s2
We see there are only 2 electrons in the outer set, and they meet the rules, since it is an s-subshell.
For a harder one, let's do Pb.
[Xe] 6s2 4f14 5d10 6p2
How many valence electrons are there?
If you got 4, you are correct. This is because there are 2 from the s-subshell, and 2 from the p-subshell. We do not count the f and d, because they are FILLED.
And that is all for this short section! :)
No comments:
Post a Comment