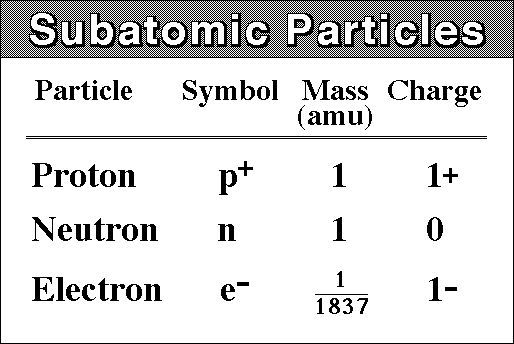
Neutrons have a neutral charge, protons have a positive charge, and electrons have a negative charge. The neutrons and protons are in the nucleus, but the electrons are actually surrounding the nucleus in energy shells.
When an atom is neutral, it means that there is no net charge. This also means that the number of protons will equal to the number of electrons.
To find the number of protons, simply look at the atomic number of the element.
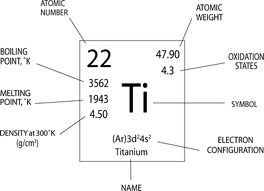
That means, if you add a proton to an element, it will become a totally different element.
Say, we have 4 protons in the nucleus of some element. This would be the element Beryllium. If we added a proton, then our element would change to Boron.
What if an atom does have some kind of charge? Then, it is called an ion. Atoms can gain or lose electrons by accepting or giving electrons to other atoms. If we are given the number of protons and the charge, we can find the number of electrons by
proton - charge
A negatively charged ion is called an anion. An anion has more electrons than protons, because non-metals tend to gain electrons.
In contrast, a positively charged ion is called a cation. This is when there are fewer electrons than protons. Metals are positively charged and tend to lose electrons.

The mass number is the total number of protons and neutrons, or it can also be found by rounding the atomic mass number to the nearest whole number. To calculate the number of neutrons in an atom, we can use the mass number and subtract from it the number of protons.
So: Mass number - atomic number (number of protons) = number of neutrons
Atomic mass is different from the mass number. The atomic mass is the average mass of an element's isotopes. They have decimal values because they are averages; however, the mass number is just a rounded number of the atomic mass.
If you add a neutron into the atom, you will get a heavier version of the element.
Now, let's try an example.
How many protons, electrons, and neutrons are in Co?
proton: 27
electron: 27
neutron: 59-27=32
An isotope of an element has the same atomic number, but a different number of neutrons or atomic mass.
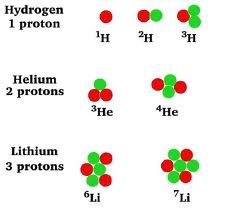
Remember back in around December, when we used the atomic mass as the molar mass?
Well, now we can understand that this mass is really an average of value of a combination of isotopes.
Let's try to find the average atomic mass, given the data of naturally occurring isotopes.
Ne-20 (90.02%) Ne-21 (0.26%) Ne-22 (9.72%)
All we have to do, is just multiply the percentages to the masses of each of the isotopes and add them up.
So: 0.9002 X 20 + 0.0026 X 21 + 0.0972 X 22 = 20.197
We have 2 sig figs, so we will get the final answer of 20.
Now here are a few links for practice:
http://cmsweb1.loudoun.k12.va.us/52820831134912597/lib/52820831134912597/Atoms%20and%20Atomic%20Theory/Homework/ws.atomic.20structure.20set.pdf
http://www.scribd.com/doc/3370461/atomic-structure-worksheet
Here's a video:
No comments:
Post a Comment