Electronic configuration is a notation that describes the orbitals in which electrons occupy, and it also gives the total number of electrons in each orbital.
In other words, it is just another way of showing which level electrons are in, just like Bohr's model.
For example, the electronic configuration for an element like carbon would be:
1s2 2s2 2p2
Now don't worry if that confuses you, because by the end of this blog, you'll know what they mean!
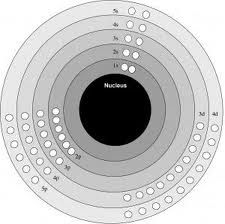
According to scientist Niels Bohr, electrons existed in specific energy states, and when an electron absorbs or emits energy, it can move from one level to another.
The scientific definition of an energy level is the maximum amount of energy an electron can hold.
When electrons are in "ground state", it means that all the electrons are in their lowest possible level. But when they are in an "excited state", it means that one or more of the electrons are in energy levels other than the lowest available level.
To write electron configurations, you should know these things:
1. Orbital - a region of space occupied by the electron
2. Shell - the set of all orbitals in the same energy level
3. Subshell - the set of orbitals of the same type.
4. There are four types of subshells (s, p, d, f)
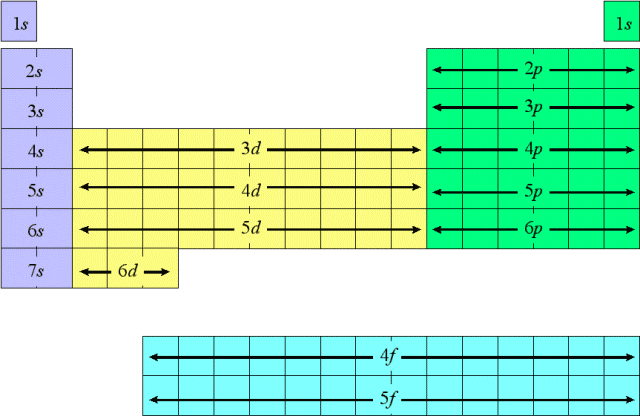
s is like the starting point. In the very first energy level, only s-types exist. Since there are in energy level one, we can say they are 1s.
In the second energy level, s and p types exist. They are in energy level 2, so they are 2s and 2p.
In the third, s, p and d types exist (3s, 3p, 3d), and in the fourth, s, p, d, and f exist (4s, 4p, 4d, 4f).
In every orbital, there are 2 electrons.
An s-type consists of one orbital (2 electrons)
p consists of 3 orbitals (6 electrons)
d consists of 5 orbitals (10 electrons)
f consists of 7 orbitals (14 electrons)
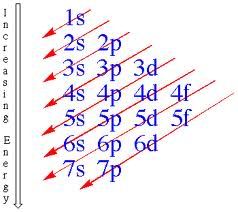
The order of writing the levels are like the picture above. Arrange the orbitals like a descending staircase, with 1s at the top.
However, in a ground state, we must remember that we can only move on to the next level when the orbitals are full. Therefore, 1s2 should be at the top. The next step in the staircase would be 2s2 2p6.
Here is a good video of electron configuration. Unfortunately, the maker of the video disabled embedding, so here's a link.
http://youtu.be/xH1k1dtgiVY
Here's a good practice worksheet; however, you will be able to do the ones that ask for abbreviated configurations after you read the next blog!
http://misterguch.brinkster.net/PRA014.pdf
No comments:
Post a Comment