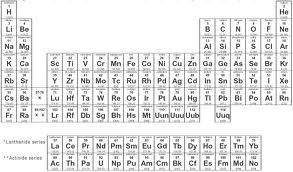
In 1869, Dmitri Mendeleev listed the elements according to the masses and showed that certain properties recur, breaking the list into rows (periods) and columns (groups). He left gaps in his periodic table, and suggested those elements had yet to be discovered. He could also predict the properties and characteristics of these undiscovered elements.


Now, the modern periodic table is organized in atomic number. The Periodic Law suggests that properties of elements recur periodically when elements are arranged in atomic number.
The families (or groups or columns) include:
Alkali metal, alkaline earth metals, halogens, and noble gases.
The rows underneath the table are called Lanthanides (starting with Lanthanum) and Actinides (starting with Actinium). These rows are considered as inner transition metals. The main transition metals are in between the metals and non-metals, and the staircase starting in group 3 signifies metalloids, which have both metal and non-metal properties.
Metals are opaque and shiny. They are good conductors of heat and electricity, and can be hammered into sheets (malleable) or drawn into wires (ductile). They are usually solids at room temperature (with Mercury being the exception), and they tend to lose electrons.

Non-metals are gases and liquids at room temperature. They are poor conductors of heat and electricity. Some non-metals are brittle solids, and are dull in appearance and opaque.

Semiconductors are non-metals with electric conductivity. They are also called metalloids, and their properties resemble metals more than non-metals. The difference is that metal conductivity decreases with increasing temperature, but semiconductor conductivity increases with increasing temperature.

Here's another summary:
http://hyperphysics.phy-astr.gsu.edu/hbase/pertab/metal.html
No comments:
Post a Comment