Some of the patterns that we will discuss include
1. Metallic properties
2. Atomic Radius
3. Ionization energy
4. Electronegativity
5. Reactivity
6. Ion charge
7. Melting/boiling point
8. Density
First, let's start with metallic properties.
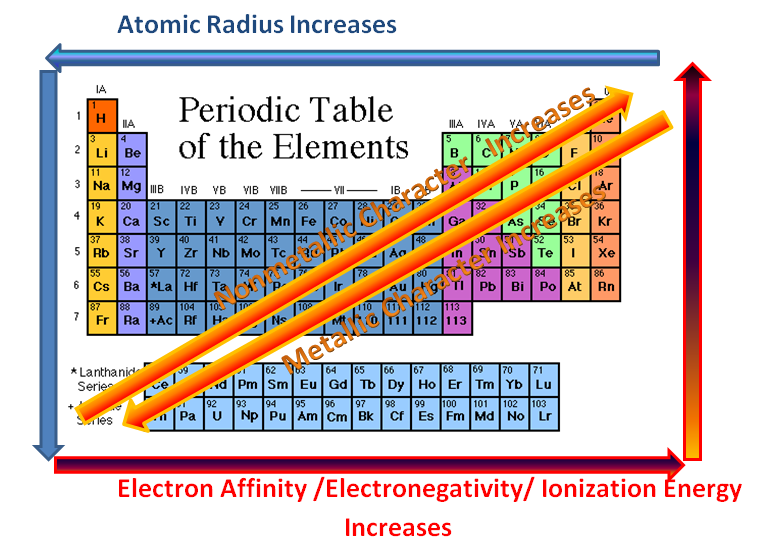
Although this one seems obvious, it is still good to point it out. When moving from left to right in the periodic table, the properties of the elements change from metallic to non-metallic.
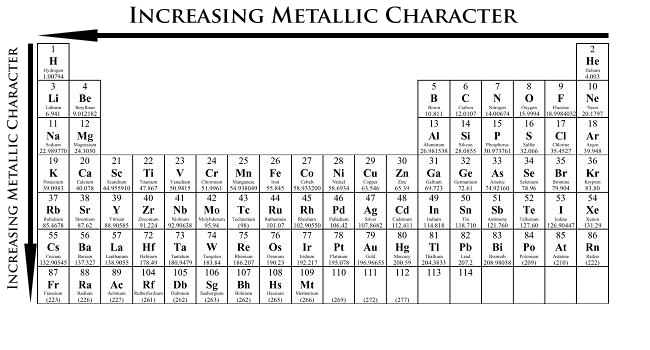
Also, when going down a family (column), elements become more metallic, or better metals.
Secondly, let's study about the atomic radii.
When moving from left to right, the atomic radii decreases. This is because the atomic number and the positive charge increase. The increase in atomic number means an increase in both electrons and protons, making the force of attraction much stronger, and decreasing the distance between each other.
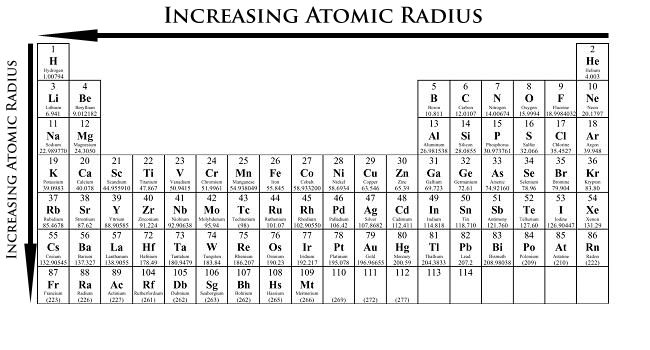
When going from up to down in a column, the radii will increase. This is because there will be more orbits taken up by the electrons. The inner electrons also repel the outer electrons, increasing the distance between the outermost electrons and the nucleus.
To recap atomic radii: left to right, decrease; up to down, increase.
Now, ionization energy. First, let's define the term. Ionization energy is the energy required to remove an electron from the neutral atom. Measured in kJ/mol, IE is basically the opposite of the atomic radius. Helium has the highest IE, and Francium has the lowest IE.
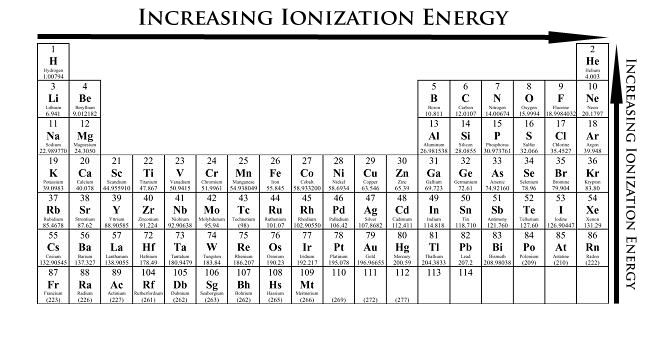
Usually, the outermost electron is removed, and so it should always be a valence electron being removed, unless it is a closed shell.
When moving left to right across a period, ionization energy increases. This is because the radii has been decreased, meaning a very strong attraction between the electrons and the nucleons (protons and neutrons). This means that it will be harder to remove an electron.
When moving up to down, the ionization energy decreases due to a larger radius. Attraction between the nucleus and the outer electrons is also decreased because there are more orbits in between blocking the way.
We can refer to removing the first electron as the first ionization energy, and removing the second as second ionization energy, etc.
Number 4, electronegativity. Definition: how much atoms want to gain electrons, or tendency to attract electrons from a neighbouring atom.
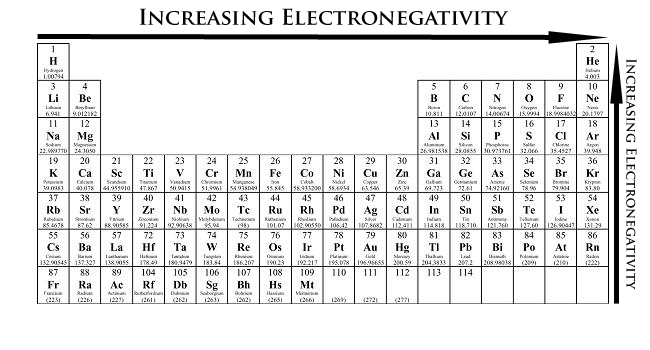
This means that if an atom has high electronegativity, it strongly attracts electrons from a neighbouring atom, and could almost "steal" an electron from its neighbour. As well, this means that the atom has a strong attraction with its valence electrons, so the electrons are harder to remove and thus has a higher ionization energy.
In the same way, lower electronegativity = lower ionization energy, because the atom does not have a strong attraction with its own valence electrons, then electrons are easier to remove.
The top right (Fluorine), except for noble gases, has the highest electronegativity.
To recap: left to right, increase in electronegativity; up to down, decrease in electronegativity.
5. Reactivity
In metals:
left to right, decreases
up to down, increases, because it is easier for electrons to be given away, meaning higher reactivity.
In non-metals, left to right, increase
up to down, decreases, because non-metals have higher electronegativity.
6. Ion Charge
The charges depend on its group.
For example,
group 1, +
group 2, 2+
group 13, 3+
group 14, 4+
group 15, 3-
group 16, 2-
group 17, 1-
group 18, 0
In the transition metals, the charges are variable.
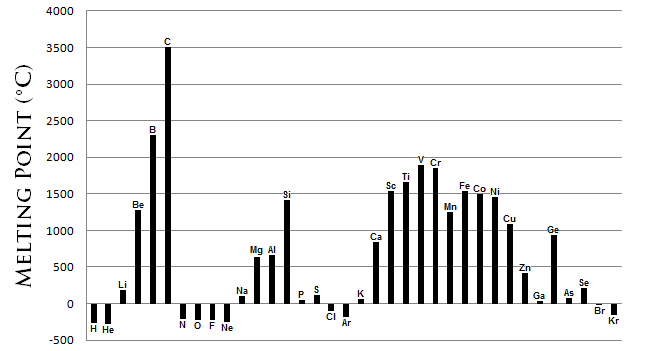
7. Melting/boiling point
The noble gases have the lowest melting points, and the elements in the center have the highest. Melting point increases from left to right, except for in the middle.
In metals, going down the group decreases the melting and boiling points.
In non-metals, going down the group increases the melting and boiling points.
8. Density
As you go down a group in the periodic table, density increases. This is because, as you go down, atomic radius increases, and volume increases, so it will be more dense.
In summary,
http://mysite.oswego308.org/schools/uploads/files/3231/ws_periodic_table_and_trends.pdf
http://butane.chem.uiuc.edu/cyerkes/Chem102AEFa07/worksheets/Worksheet%2012.pdf
Excellent information provided about periodic table with its properties like atomic radius,ionization energy and electronegativity.
ReplyDeleteLinear Programming
Wouldn't density increase as you move down the group because the mass is increasing MORE than the volume is increasing. If only the volume were increasing it would be less dense.
ReplyDeletecan i get a hoyeah?
ReplyDelete