Try to get the lowest number for indicating the double or triple bond position. An alkene has a double bond.
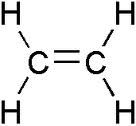
For example,
CH2 = CH - CH2 - CH3
would be butene. Notice that the name will end in -ene.
CH2 = CH2 would be ethene
The general formula is C(N)H(2N)
Some general rules for naming:
1) Find the longest chain. This will be the part that makes up the end of the name.
2) Number the carbon atoms to get the lowest number for the start of the double bond and place this number before the parent name.
3) Write the names and numbers for the side groups, assembling them alphabetically.
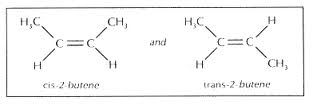
Some alkenes have the same structure, but a different geometry (or a different shape). These are called geometric isomers.
We use trans- or cis- to distinguish between these isomers.
If two adjacent carbon atoms are bonded by a double bond and have side chains on them, two chains are possible.
If the larger groups are both on the top or on the bottom, then it is cis.
If the larger groups are diagonal to each other, or across from each other, then it is trans.
If the larger groups are one on top of the other, then there are no isomers, and therefore no cis or trans.
Now, let's do alkynes. These are the same as alkanes or alkenes in terms of naming, but these just end in -yne.
Alkynes have one or more triple bond between carbons which lead to unsaturated hydrocarbons.
For example:
CH ≡ CH would be ethyne.
The general formula is C(N)H(2N-2)
There are no cis or trans forms since they are mostly the same.
Now, let's try a few examples.
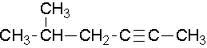
The longest chain is 6, and the position of the triple bond is 2, counting from right to left. This is because we get the lowest number possible.
First, write down 2-hexyne. There is a methyl group on the 5th position (since we are already counting right to left, we must stay consistent)
Finally, we end up with 5-methyl-2-hexyne.
Here's a video on alkene.
Alkynes:
http://herh.ccrsb.ca/staff/FarrellL/chem11downloads/org/org2.pdf
No comments:
Post a Comment