Properties of organic compounds are low melting points, with low electricity. The carbon atoms can be linked in straight lines (which are really zigzag), circular structures, or in branches. As well, they can be linked in single, double or triple bonds.
First, we will start with alkanes. These are unbranched, straight chain compounds that only contain H and C. These are all hydrocarbons that are bonded together in single bonds. The names will end in "-ane" because they belong to the "alkane" group.
For the molecular formula C2H6, the full structure is:
H H
| |
H - C - C - H
| |
H H
As you see, the two carbons are bonded together, and the remaining bonds are with hydrogens.
The formulas for regular alkanes are:
Methane CH4 Hexane C6H14
Ethane C2H6 Heptane C7H16
Propane C3H8 Octane C8H18
Butane C4H10 Nonane C9H20
Pentane C5H12 Decane C10H22
For alkanes, the general formula is C(N)H(2N+2).
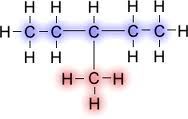
When hydrocarbons have side branches, they are called substituted hydrocarbons or branched hydrocarbons. (If you refer to the diagram above, this means that one of the Hydrogens surrounding the C is replaced with, say, a methane)
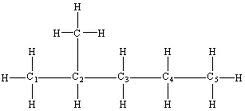
Carbon has 4 bonds. When a carbon is attached to another carbon, there are 3 hydrogens surrounding the original carbon. When carbon is attached to two other carbons, there are 2 hydrogens. When attached to 3 carbons, there is 1 hydrogen left. When carbon is attached to 4 carbons, there are no hydrogens left.
For example, if a carbon is attached to another carbon, we would get:
H
|
CH3 H - C - H
| |
H - C - H or H - C - H (the CH3 is the same as an added carbon, with 3 hydrogens
| | branching out)
H H
The CH3 is supposed to be methane, but one H got bonded with the original H that was there, and so CH3 is left.
To name this, we would say this is ethane. We will go through this more later.
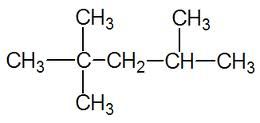
Suppose there is a name: 2-methylpentane
The methyl part is an alkyl group, which is an alkane which has lost one hydrogen atom.
The parent hydrocarbon is pentane (which is the longest straight chain, as there are 5 carbons).
The names of the alkyl groups end in "yl" because they are alkyl. If there are 2 or more of the same kind of alkyl group, use prefixes like di, tri, tetra, penta.
If more than one alkyl group (as in different kinds of alkyl groups) are present, list them alphabetically, and put the position number in front, with a dash between each group and number.
Here's a video:
http://www.arps.org/users/hs/thompsom/chemcom/unit_3/Naming_Alkanes_Worksheet_1.pdf
http://www.grossmont.edu/martinlarter/chemistry141/reference/AlkaneWorksheet.pdf
No comments:
Post a Comment