If electrons are shared equally, we say the covalent bond is non-polar. If they are shared unequally, a polar covalent bond is formed.
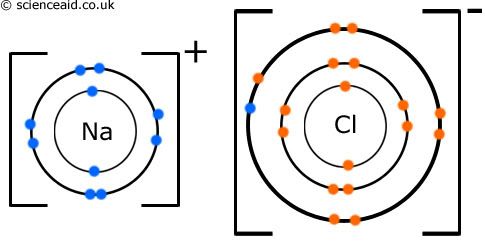
But when electrons are transferred, it is totally different, and it is an ionic bond.
Recall from the periodic table trends the term electronegativity. It is the tendency to attract electrons, with non-metals having a high number of electronegativity.
In non-polar bonding, equal sharing is observed to get a full shell. Electrons are attracted to the nuclei, and there are some in between the two atoms. The bonds are very strong, and require a high amount of energy to break them.
Intramolecular forces hold atoms of the molecule together. Intermolecular forces bond the molecules together. When melting occurs, the bond between the atoms is not broken, and only the intermolecular, weak bonds are affected.
In polar bonding, atoms with greater electronegativity pull electrons toward itself, so the shared electrons will actually be closer to this atom.
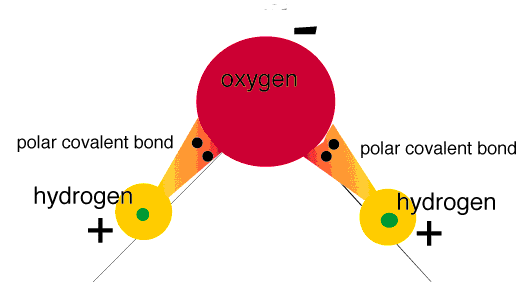
Just remember, atoms with the higher electronegativity form a PARTIAL NEGATIVE CHARGE, while the lower electronegativity atom will form a PARTIAL POSITIVE CHARGE.
Then, add an arrow to indicate which way the electrons will tend to move. (in other words, draw the arrow pointing to the partial negative atom)
E.g. What is the bond between C and O?
To do this, we will use a Table of Electronegativities. If the difference is < 0.5, it is covalent. If the difference in EN is >0.5 and <1.8, it is polar covalent. If the difference is >1.8, it is ionic.
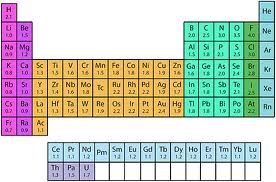
So, the EN of C is 2.55, and the EN of O is 3.44, giving a difference of 0.89, which is polar covalent.
This means our diagram would look like:
C δ+ ---------> O δ-
Here's a video:
On related videos, there are continuations of this video.
Here is some practice:
http://chemistry.sswiki.com/file/view/8.4+Review.pdf
http://www.dorjegurung.com/chemistry/IB_year1/worksheets/wkst_hybridization_shape_polarity.pdf
No comments:
Post a Comment