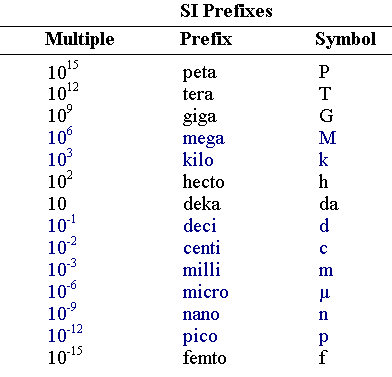
Converting units isn't hard, as long as you work at it, and understand the basic fundamentals. Converting units allows you to change different units of measurement for the same quantity.
For example: If you wanted 1L of milk, and you wanted to convert it to mL, then you can set up an equation and find out the result.
1L x 10^3mL
______ = 1 x 10^3mL
1L
You have just converted 1L into mL. Here we can see that 1L = 10^3 mL, which is the same as 1000 mL.
* Because you wanted to find out how many mL is in one Litre, when setting up your equation, make sure that the L unit is able to cancel out.
______ = 1 x 10^3mL
Watching this video will help you with your understanding of converting units:
Doing more of these questions will help strengthen your understanding on how to convert units.You can go on these websites for practice.
http://chemistry.about.com/library/metricmetric.pdf
http://serc.carleton.edu/files/mathyouneed/metric_conversion_pp.pdf
Good luck on your conversions!
No comments:
Post a Comment